The oncology drug market is experiencing a significant shift with the increasing availability of therapeutically equivalent, substitutable drugs, including biosimilars and 505(b)(2) drugs. These alternatives offer potential cost savings and expanded treatment options. However, due to their quick growth rate in the market and varying payer preferences, their integration into existing electronic health record (EHR) systems presents challenges, necessitating new workflows to facilitate their adoption.

Flatiron Health has been at the forefront of addressing these challenges with its biosimilar substitution feature within OncoEMR®, which has successfully streamlined the adoption of biosimilars in oncology practices since 2022. However, managing 505(b)(2) drugs poses unique difficulties, prompting Flatiron to expand its existing substitution functionality to support these medications.
Tennessee Oncology, a large independent community oncology practice with 71 full-time medical oncologists, emerged as an early adopter of this innovative feature. As one of the nation's largest community-based cancer centers, Tennessee Oncology's experience in implementing the ingredient drug substitution feature provides valuable insights into the practical benefits and impact of Flatiron's solution, which is the first of its kind to market.
The Challenge
Managing 505(b)(2) drugs in oncology practices presents several complexities:
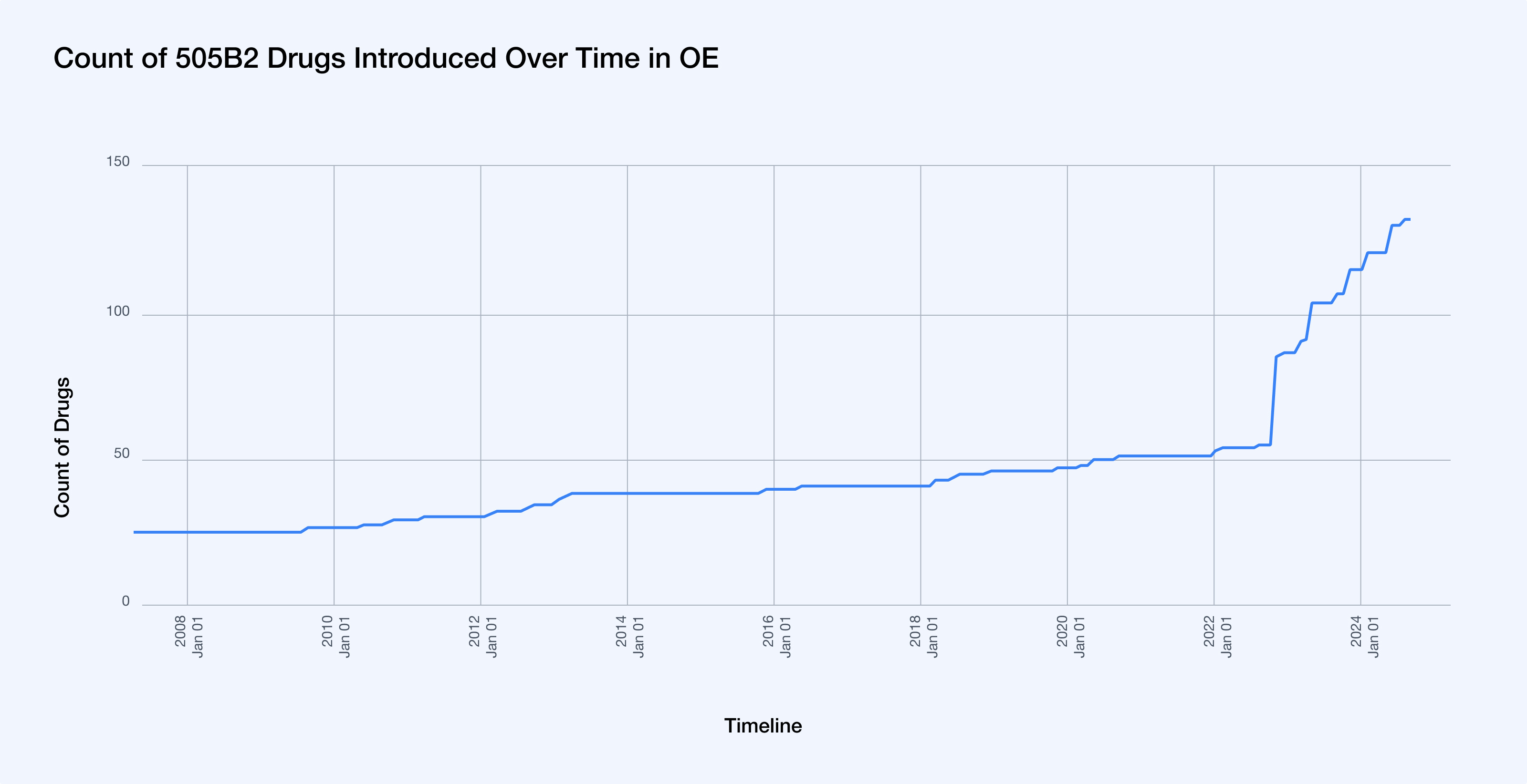
- Tracking new 505(b)(2) market entries: While the 505(b)(2) pathway has been available since 1984, it has seen a surge in activity given the advantages it affords manufacturers including its quicker pathway to approvals and higher potential for exclusivity. The recent surge can be attributed to a significant policy change: in 2022, the Centers for Medicare & Medicaid Services (CMS) decided that newly approved 505(b)(2) products not rated as therapeutically equivalent would each be assigned a unique billing and payment code.1 This decision made the 505(b)(2) pathway even more attractive to manufacturers, as it potentially allows for separate reimbursement and pricing strategies for these products, further driving the increase in 505(b)(2) drug applications.2 From 2017-2020, over half of all NDA approvals went through the 505(b)(2) pathway.3 And to further illustrate this trend, the number of 505(b)(2) drugs in the formulary in OncoEMR® (OE) has increased by 138.2% since 2021. This requires significant and thorough formulary management on behalf of practices to stay up to date with the latest drugs in market.
-
Identifying appropriate substitutions: Without approved therapeutic equivalence between the 505(b)(2) drugs and their respective originators, practices have the outsized responsibility of identifying and managing suitable alternatives without established standards or guidance.
-
Varied administration differences: Unlike biosimilars, which have the same indication and administration guidance as their originators, 505(b)(2)s can vary from their originators in the respective fluid type and amount, duration, and administration instructions, impeding a simple 1:1 substitution between drugs.
-
Regimen management overhead: To support substitutions, practices complete a variety of workarounds, including reactive, manual 1:1 substitutions per order per patient, creating duplicate regimens with each target 505(b)(2), or adding multiple 505(b)(2)s per regimen. This requires significant attention to ensure correct ordering, with high risk for human error.
-
Ensuring compliance with payer requirements: Due to differences in indication, administration, price and generic alternatives, payers reimburse originator and 505(b)(2) drugs at varying rates. Drug preferences can vary significantly between payers and even among health plans within the same payer. To complicate matters, these preferences can change on a quarterly basis, adding a considerable burden to the adjustment of regimens, orders, and formularies.
-
Potential workflow disruptions: Substituting 505(b)(2)s is an extensive and manual process, from tracking drug orders, keeping the formulary up to date, and getting proper authorizations. Without a streamlined system, providers may face delays and inefficiencies in prescribing and administering practice and payer-preferred drugs, potentially impacting patient care.
The absence of a standardized identification method for 505(b)(2) drugs and their substitutable counterparts necessitated an innovative approach to achieve clinically accurate, scalable, and feasible substitutions.
The Solution
Flatiron's Ingredient Drug Substitution for 505(b)(2) feature provides a comprehensive solution to these challenges by automatically substituting regimen drug orders according to practice-driven preferences. Its primary benefits include:
-
Streamlined, automated workflow: By integrating substitution options directly into the EHR, the feature minimizes disruptions to clinical workflows while ensuring compliance with practice and payer preferences.
-
Customizable substitution groups: Practices can create and manage custom substitution groups tailored to their formulary availability, needs, and preferences. Groups are built to automatically manage differences between products, including fluid type and amount, duration, and administration instructions.
-
Payer-specificity: The feature enables practices to set practice-specific and insurer preferences, which ensures that substitutions align with coverage requirements and preferences.
-
Reduction in regimen management: With the creation of comprehensive substitution groups that incorporate practice and insurer preferences, practices no longer need to manage duplicate regimens, edit regimens, or complete manual workarounds to ensure adherence.
-
Scalable logic to future-proof against market growth: The product is scalable to account for rapid growth of 505(b)(2)s in market, enabling practices to centrally add and edit drugs within groups as new drugs are available, and efficiently manage practice and payer preferences as these change.
The Impact
The implementation of Flatiron's Drug Substitution for 505(b)(2) feature at Tennessee Oncology has yielded significant positive outcomes. This success demonstrates its potential to significantly reduce administrative burdens across a broader range of community oncology practices:
-
Increased accuracy in adhering to practice and insurer preferences: Comparing flowsheets ordered with 505(b)(2)s approximately two months before the Ingredient Drug Substitution functionality was released to Tennessee Oncology and two months after, the practice saw a 16-point increase in order accuracy (alignment to practice and insurer preferences). Furthermore, 80% of flowsheets with substitutions were accurate at the point of ordering, and necessitated no manual changes to align with practice and insurer preferences.4
-
Reduction in manual interventions and substitutions: Feedback from Tennessee Oncology revealed a marked decrease in reactive order corrections. Kaitlyn Swords, RN, and Tracy Valrie, Drug Authorization Manager, noted fewer manual interventions to align orders with practice and insurer preferences. As Valrie stated, "It's cut down on the number of requests we've gotten for the groups the team has put in place (Bendamustine, Bortezomib, Cyclophosphamide, Docetaxel, Pemetrexed)." Quantitatively, Tennessee Oncology has experienced an 80% decrease in manual substitutions and post-ordering changes to drug orders.
-
Reduction in time-spent managing formulary, regimens, and manual substitutions: As teams spend less time tracking new 505(b)(2) market entries, updating regimens, identifying appropriate substitutions, and ensuring compliance with payer requirements, they can ultimately focus more on patient care. Leigh Wrisner, Clinical Nurse Specialist with the Onco Regimen Drug Team, quantified the time savings: "It's difficult to put a number on it because of the workarounds we had in place, but I'd estimate 4-8 hours/week saved from doing manual swaps, especially when needing to transfer from one practice-preferred drug to another."
-
Increased speed to treatment and positive impact on patient care: In an interview, Ted Arrowsmith, MD, Executive Vice President of Therapeutics at Tennessee Oncology and Medical Director of Pathways at OneOncology, emphasized the transformative impact of this new feature in accelerating treatment initiation for patients with highly treatable lymphomas: "What's really going to change things is being able to avoid delays when starting a new regimen - that's the real key. Some of these drugs (cyclophosphamide, bendamustine) for highly treatable lymphomas - you want to get the patient started on treatment as soon as possible, and this functionality speeds up that process and removes the delays with swaps and pre-auths. There's also less risk for error."
Conclusion
Flatiron's Ingredient Drug Substitution for 505(b)(2) offers a valuable solution for community oncology practices of all sizes. By automating routine tasks, reducing administrative burdens, and supporting data-driven insights, this feature empowers clinicians to focus on delivering high-quality care to their patients.
The experience of Tennessee Oncology demonstrates the tangible benefits of this technology, including time savings, improved treatment initiation, and reduced manual interventions.
2- https://www.pharmacytimes.com/view/understanding-the-505-b-2-pathway
4 - Pre-release data collection timeframe was 5/18/24-7/18/24, and post-release data collection time frame was 7/19/24-9/10/24. Flowsheet accuracy is measured by the number of regimens created with a specific 505(b)(2) drug, where no 505(b)(2) drugs are later deleted, added or edited.



