Clinical research has always been a cautious and time-consuming affair. The stakes are high, and getting it wrong is not an option. But the slow pace, complexity and operational burden involved in clinical research today has been a constant source of frustration for stakeholders: from sites to sponsors, healthcare providers, policymakers, and ultimately patients.
About a decade ago, a fresh new approach emerged with real-world evidence (RWE), based on real-world data (RWD) captured at the point of care.[1] Spurred on by the near-universal adoption of electronic health records,[2] rapid advances in data technology and infrastructure and the FDA’s 21st Century Cures Act, RWE breathed new life into clinical research.
Where are we now? Biopharma companies are using RWE at every point of the value chain: from assessing unmet needs to informing study designs and monitoring outcomes. But it rarely plays a central role in regulatory submissions. Only 1% of Phase IV trials currently incorporate RWD, and barely more than 3% of all RWE studies have anything to do with drug-specific effectiveness, discontinuation rates, or adverse event rates.[3]
A new breed of RWE studies, prospective studies, is starting to change that equation.
What are prospective studies?
Most RWE studies today rely on data that’s already been captured in the course of routine care: in electronic health records, insurance claims, patient surveys, or from patient, product and disease registries. Those studies focus on analyzing existing data, not capturing new data elements. It’s actually one of their biggest advantages: Since the data already exists, the impact on clinical sites is minimal and lead time for sponsors can be greatly reduced.
But what makes RWE so compelling is also limiting its potential applications. RWD in those studies is retrospective data, not prospective data. Researchers use it to analyze what happened in the past, not what might happen in the future.
Prospective studies are designed to intentionally collect new data on top of existing RWD: more detailed, specific, and complete data that is critical to the study’s objectives such as adverse events, clinical-trial-like outcome measures, patient-reported outcomes and non-standard genomic data.[4] The ability to tailor the data collection to the study’s objectives makes this approach well suited for post-marketing studies, or to augment the control arm of an RCT study.
They typically don’t require costly new technology or much training at the site, and they’re generally executed across a network of sites that already have the technical infrastructure and the know-how, to accommodate the small amount of extra data collection during routine clinical practice. When these studies are well designed and well integrated into familiar point-of-care technology, sites and patients are more likely to participate.
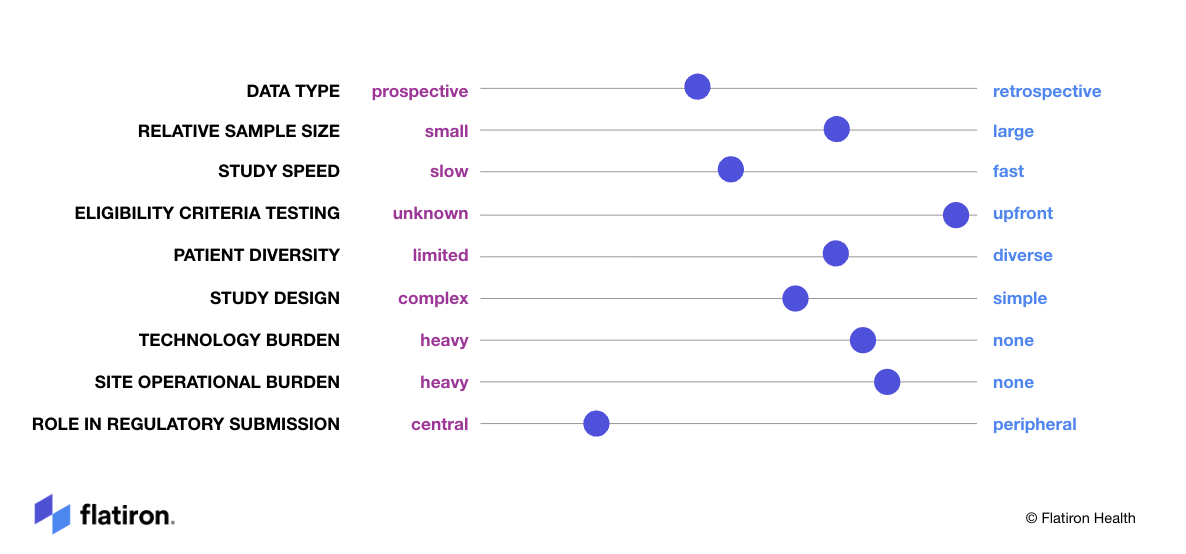 Figure 1: Overview of prospective studies on various dimensions critical to clinical research success
Figure 1: Overview of prospective studies on various dimensions critical to clinical research success
What results can you expect from prospective studies?
A good example of the benefits of Flatiron's approach to prospective studies is the Prospective Clinico-Genomic (PCG) Study.[5] A partnership between Flatiron Health, Foundation Medicine and Genentech, the PCG study ran across 24 sites for about 18 months and enrolled nearly a thousand patients with a documented diagnosis of metastatic non-small cell lung cancer or extensive stage small cell lung cancer.
The research team relied on a suite of tools already present at the sites to help them identify eligible patients, track patient status, collect data from the EHR and respond to queries. No separate electronic data capture (EDC) system was needed, and the physicians spent a minimal amount of additional time with patients enrolled in the study.
Research shows that only about 6% of cancer patients ever take part in a clinical treatment trial, with a wide difference between patients enrolled in NCI-designated comprehensive cancer centers (19%) and those enrolled in community cancer programs (4%).[6] When left unchecked, that selection bias can seriously hinder the validity of clinical research.
By comparison, one of the practices involved in the PCG study was able to enroll over 40% of its eligible patient population—a giant win for patient access and representation.
The promise ahead
This is a huge opportunity for biopharma companies. With the right partners and attention to data and methodological integrity, prospective studies can reach beyond the limitations of today’s RWE studies and expand the role of RWD across the entire drug development cycle, including regulatory submissions.
Consider your current research portfolio. Can you see ways to incorporate prospective data into the mix? Perhaps a prospective study could help you execute a post-marketing study you thought you didn’t have time for, or augment the control arm of a planned RCT at a fraction of the cost and without any degradation in data quality.
Only 17% of RWE studies use prospective cohorts today,[7] so the road ahead is long. But the field has evolved considerably in recent years, and with the right investments in technology, data and infrastructure on the front end, and in analytics and modeling on the back end, the future is bright for prospective studies in clinical research.
1 The FDA defines RWD as “data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources” and RWE as “clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD.”
2 National Trends in Hospital and Physician Adoption of Electronic Health Records. Office of the National Coordinator for Health Information Technology (2021)
3 Getting real: who is leading the real-world data charge with clinical trials? Clinical Trials Arena (2022)
4 The case for including microbial sequences in the electronic health record. Nature Medicine (2023)
5 Prospective Clinico-Genomic Study. Flatiron (2021)
6 Nationally representative estimates of the participation of cancer patients in clinical research studies according to the commission on cancer. Journal of Clinical Oncology (Oct 2021)
7 Use of real-world evidence in economic assessments of pharmaceuticals in the United States. J Manag Care Spec Pharm. (2021)